Software and AI driven smart applications offer exciting solutions to health issues but challenge existing regulatory pathways. But, not all medical software is SaMD, and therefore not all need go through the FDA regulatory approval process. To determine if medical software is truly SaMD just answer the following; does the medical software diagnose, treat, drive or inform clinical management? If the answer is yes to any one of these, then the medical software must go through the regulatory approval process before market introduction and use.
The iterative nature, pace, and volume of SaMD innovation has pressed the FDA to create a new regulatory paradigm that serves the people while still preserving safety. The FDA’s “Digital Health Software Pre-certification (Pre-Cert) Program” uses a Total Product Lifecycle (TPLC) approach with four main components; 1) Excellence Appraisal, 2) Review Determination, 3) Streamlined Review, and 4) Real-World Performance to ensure patient safety and product effectiveness. [1] The goal of the Software Precertification program is to have tailored, pragmatic, and least burdensome regulatory oversight. It’s designed to assess organizations in order to establish trust that they have a culture of quality and organizational excellence, that they can develop high quality SaMD products, and can leverage transparency across the entire lifecycle of SaMD, and leverage unique software post-market opportunities to verify the continued safety, effectiveness, and performance of SaMD in the real world. [1] (Figure1).

Figure1: FDA Pre-Cert v1.0 ( source: FDA )
To streamline the review process, risk categorization is an early and primary consideration for this new expedited process. SaMD risk categorization framework plays a function similar to existing medical device classification (e.g., Class 1, 2, 3) by stratifying submissions based on risks posed to patient safety. [2]
(Figure 2).
In the SaMD Risk Framework (Figure 2) the rows indicate the criticality of the health condition the SaMD is designed to address, while the columns indicate the significance of the information the device provides in the health care decision making process. Based on Pre-Cert level and SaMD low risk level (Category 1) a product could skip an in-depth review all together and go straight to market, while a Category II may skip review or undergo an expedited review process. [3]

Figure 2: IMDRF type of SaMD Risk Categorization Framework (source: International Medical Device Regulators Forum)
An organizations FDA Pre-Cert eligibility (Excellence Appraisal) will be determined based on a company’s ability to demonstrate a culture of quality and organizational excellence (CQOE). The overall success of organizations participating in the complete, new SaMD regulatory process will be predicated on their commitment to follow the tenets of mutual trust, transparency, and sharing of RWD in order for the FDA to monitor and verify all SaMD’s safety post-market.
Many questions remain regarding the FDA’s transformation of the medical device regulatory process to accommodate SaMDs. If you are interested in getting your SaMD successfully through the regulatory process and gain reimbursement from potential payers, Da Vinci Health Group would be happy to help you navigate these healthcare waters. With global health regulatory and payment expertise in medical devices, digital health, and biologics we are poised to accelerate market adoption of your SaMD.
References
[1] FDA, “Developing a Software Precertification Program: A working Model”, v1.0-January 2019
[2] Bakul Patel and the Software as a Medical Device Working Group, “Software as a Medical Device: Clinical Evaluation, “International Medical Device Regulators Forum, August 5, 2016, p. 27, Link
[3] FDA, “Digital Health Innovation Action Plan, “accessed March 24, 2019, Link
Navigating Payment for Promising Digital Health Solutions

The Promise of Digital Health Solutions meets the Reality of Healthcare Decision Makers
Digital health technologies have exploded over the past few years. There is much hope and excitement around the potential improvements digital solutions can bring to healthcare. Within the digital health world there are major subcategories such as mHealth(mobile), Health IT, Big Data, sensors, personal genomics, and telemedicine for example (Figure 1).
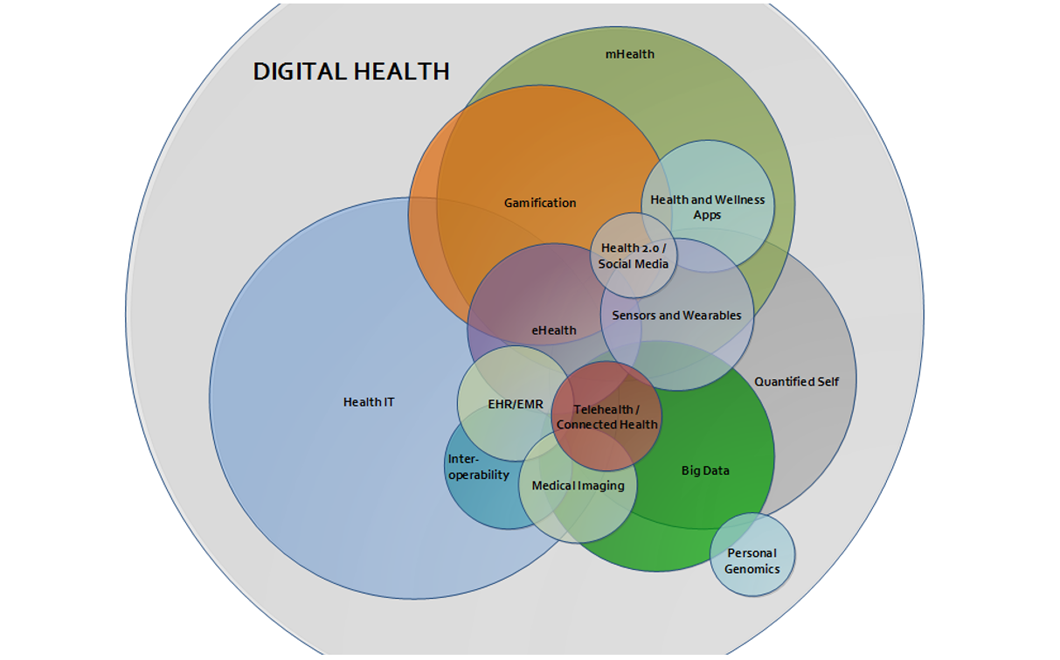
Figure 1: digital health landscape (source: IMT )
Most digital health solutions are attempting to solve vexing healthcare delivery problems, flow of health data, or to directly improve patient lives. Therefore, it is important to understand how these technologies will interface with or replace established healthcare processes and meet the demands of healthcare regulations by country. Academics and regulators are still struggling to define digital health, but consensus encompasses prevention, diagnosis, treatment, and remote management of disease. [1,2,3]. A wide range of Digital Health products already exist, and an extensive list of products described in peer-reviewed literature was presented by Iyawa et al. [4] Whether these meet the patients’, providers’, and payers needs are key to their success. This means all developing digital health technologies need to consider their value to patients, providers, and payers, and then validate that value by documenting and publishing objective evidence to back up their claims, otherwise it's just an idea. In the eyes of healthcare decision makers like Medicare, NICE, HAS, and the like something is only worth paying for, if there is objective evidence of its value compared to existing standards. The same can be said for hospitals, clinics, and physicians in the US, who now have financial risk for patient outcomes and cost efficiencies [5]
Dexcom® is a shining example of breakthrough technology that invested in producing clinical and health economic evidence to demonstrate its value compared to standard diabetic interventions. They also did the right thing by engaging Medicare early on in product development to ensure they were on the right track for the proof needed for adequate reimbursement. The result of proof of value planning is their COMISAIR Study which showed their CGM technology drives A1C reductions not the delivery system. [6] For patients, Dexcom® gave them freedom and peace of mind by proving Dexcom’s CGM® reduces median time spent in hypoglycemia by 79% at night. [7] For providers, Dexcom® has a well thought out reimbursement payment process and a patient-provider communication loop and report that fulfills requirements of key quality measures.
All existing systems and Digital Health solutions have pros and cons. With experience in global strategic market access planning and reimbursement of digital solutions, Da Vinci Health Group would be happy to help with any questions you may have about Digital Health market adoption and payment.
References
[1] Phil Baumann, the definition of digital health, 2015
[2] Paul Sonnier, Definition of Digital Health, 2013
[3] Brennan Spiegel, Three Keys to Unlocking Data-Driven Health Care, 2017
[4] Iyawa et al., Digital health innovation ecosystems: From systematic literature review to conceptual framework, 2016
[5] Digital Health Study Physicians’ motivations and requirements for adopting digital clinical tools (2016, AMA)
[6] Soupal J, Petruzelkova L, Flekac M, et al. Comparison of Different Treatment Modalities for Type 1 Diabetes, Including Sensor-Augmented Insulin Regimens, in 52 Weeks of Follow-Up: A COMISAIR Study. Diabetes Technol Ther. 2016;18(9):532-538.
[7] Beck RW, Riddlesworth T, Ruedy K, et al. Effect of Continuous Glucose Monitoring on Glycemic Control in Adults With Type 1 Diabetes Using Insulin Injections: The DIAMOND Randomized Clinical Trial. JAMA. 2017;317(4):371-378.